David Orenstein | The Picower Institute for Learning and Memory
2025-02-06 16:40:00
news.mit.edu
A new technology developed at MIT enables scientists to label proteins across millions of individual cells in fully intact 3D tissues with unprecedented speed, uniformity, and versatility. Using the technology, the team was able to richly label large tissue samples in a single day. In their new study in Nature Biotechnology, they also demonstrate that the ability to label proteins with antibodies at the single-cell level across large tissue samples can reveal insights left hidden by other widely used labeling methods.
Profiling the proteins that cells are making is a staple of studies in biology, neuroscience, and related fields because the proteins a cell is expressing at a given moment can reflect the functions the cell is trying to perform or its response to its circumstances, such as disease or treatment. As much as microscopy and labeling technologies have advanced, enabling innumerable discoveries, scientists have still lacked a reliable and practical way of tracking protein expression at the level of millions of densely packed individual cells in whole, 3D intact tissues. Often confined to thin tissue sections under slides, scientists therefore haven’t had tools to thoroughly appreciate cellular protein expression in the whole, connected systems in which it occurs.
“Conventionally, investigating the molecules within cells requires dissociating tissue into single cells or slicing it into thin sections, as light and chemicals required for analysis cannot penetrate deep into tissues. Our lab developed technologies such as CLARITY and SHIELD, which enable investigation of whole organs by rendering them transparent, but we now needed a way to chemically label whole organs to gain useful scientific insights,” says study senior author Kwanghun Chung, associate professor in The Picower Institute for Learning and Memory, the departments of Chemical Engineering and Brain and Cognitive Sciences, and the Institute for Medical Engineering and Science at MIT. “If cells within a tissue are not uniformly processed, they cannot be quantitatively compared. In conventional protein labeling, it can take weeks for these molecules to diffuse into intact organs, making uniform chemical processing of organ-scale tissues virtually impossible and extremely slow.”
CuRVE and eFLASH protein labeling
Video: The Picower Institute
The new approach, called “CuRVE,” represents a major advance — years in the making — toward that goal by demonstrating a fundamentally new approach to uniformly processing large and dense tissues whole. In the study, the researchers explain how they overcame the technical barriers via an implementation of CuRVE called “eFLASH,” and provide copious vivid demonstrations of the technology, including how it yielded new neuroscience insights.
“This is a significant leap, especially in terms of the actual performance of the technology,” says co-lead author Dae Hee Yun PhD ’24, a recent MIT graduate student who is now a senior application engineer at LifeCanvas Technologies, a startup company Chung founded to disseminate the tools his lab invents. The paper’s other lead author is Young-Gyun Park, a former MIT postdoc who’s now an assistant professor at KAIST in South Korea.
Clever chemistry
The fundamental reason why large, 3D tissue samples are hard to label uniformly is that antibodies seep into tissue very slowly, but are quick to bind to their target proteins. The practical effect of this speed mismatch is that simply soaking a brain in a bath of antibodies will mean that proteins are intensely well labeled on the outer edge of the tissue, but virtually none of the antibodies will find cells and proteins deeper inside.
To improve labeling, the team conceived of a way — the conceptual essence of CuRVE — to resolve the speed mismatch. The strategy was to continuously control the pace of antibody binding while at the same time speeding up antibody permeation throughout the tissue. To figure out how this could work and to optimize the approach, they built and ran a sophisticated computational simulation that enabled them to test different settings and parameters, including different binding rates and tissue densities and compositions.
Then they set out to implement their approach in real tissues. Their starting point was a previous technology, called “SWITCH,” in which Chung’s lab devised a way of temporarily turning off antibody binding, letting the antibodies permeate the tissue, and then turning binding back on. As well as it worked, Yun says, the team realized there could be substantial improvements if antibody binding speed could be controlled constantly, but the chemicals used in SWITCH were too harsh for such ongoing treatment. So the team screened a library of similar chemicals to find one that could more subtly and continuously throttle antibody binding speed. They found that deoxycholic acid was an ideal candidate. Using that chemical, the team could not only modulate antibody binding by varying the chemical’s concentration, but also by varying the labeling bath’s pH (or acidity).
CuRVE and eFLASH protein labeling
Video: The Picower Institute
Meanwhile, to speed up antibody movement through tissues, the team used another prior technology invented in the Chung Lab: stochastic electrotransport. That technology accelerates the dispersion of antibodies through tissue by applying electric fields.
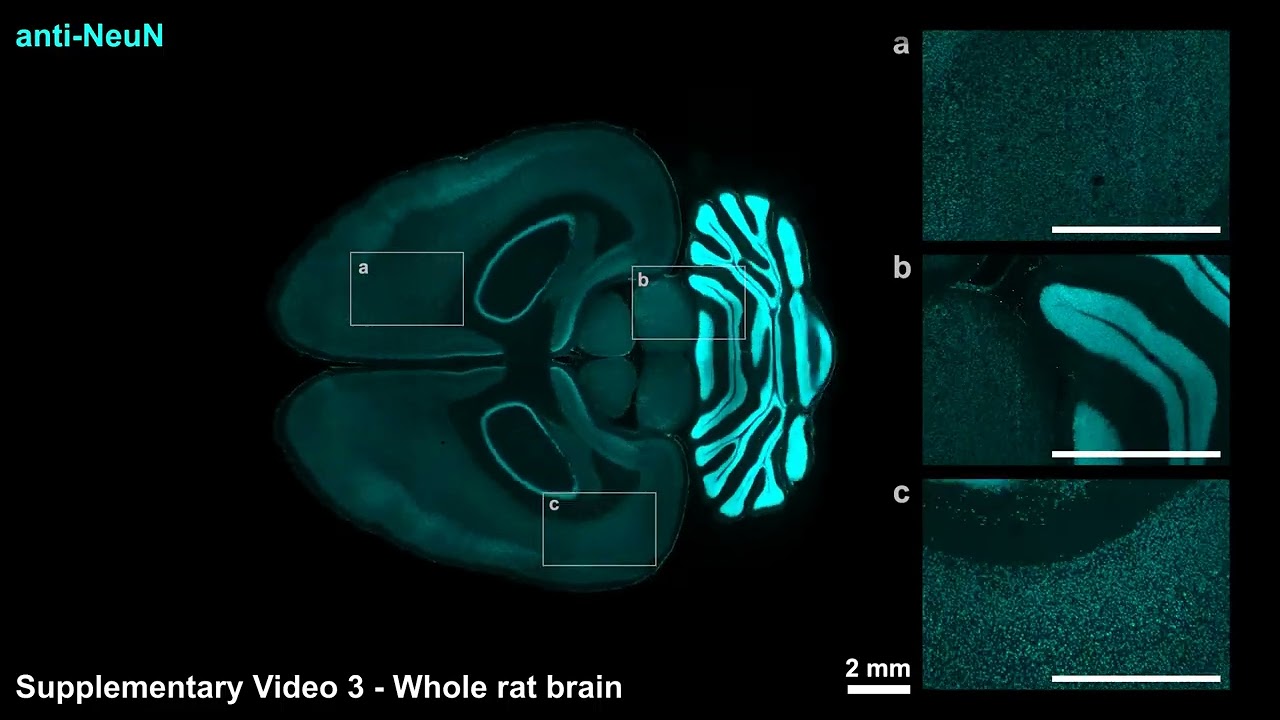
Implementing this eFLASH system of accelerated dispersion with continuously modifiable binding speed produced the wide variety of labeling successes demonstrated in the paper. In all, the team reported using more than 60 different antibodies to label proteins in cells across large tissue samples.
Notably, each of these specimens was labeled within a day, an “ultra-fast” speed for whole, intact organs, the authors say. Moreover, different preparations did not require new optimization steps.
Valuable visualizations
Among the ways the team put eFLASH to the test was by comparing their labeling to another often-used method: genetically engineering cells to fluoresce when the gene for a protein of interest is being transcribed. The genetic method doesn’t require dispersing antibodies throughout tissue, but it can be prone to discrepancies because reporting gene transcription and actual protein production are not exactly the same thing. Yun added that while antibody labeling reliably and immediately reports on the presence of a target protein, the genetic method can be much less immediate and persistent, still fluorescing even when the actual protein is no longer present.
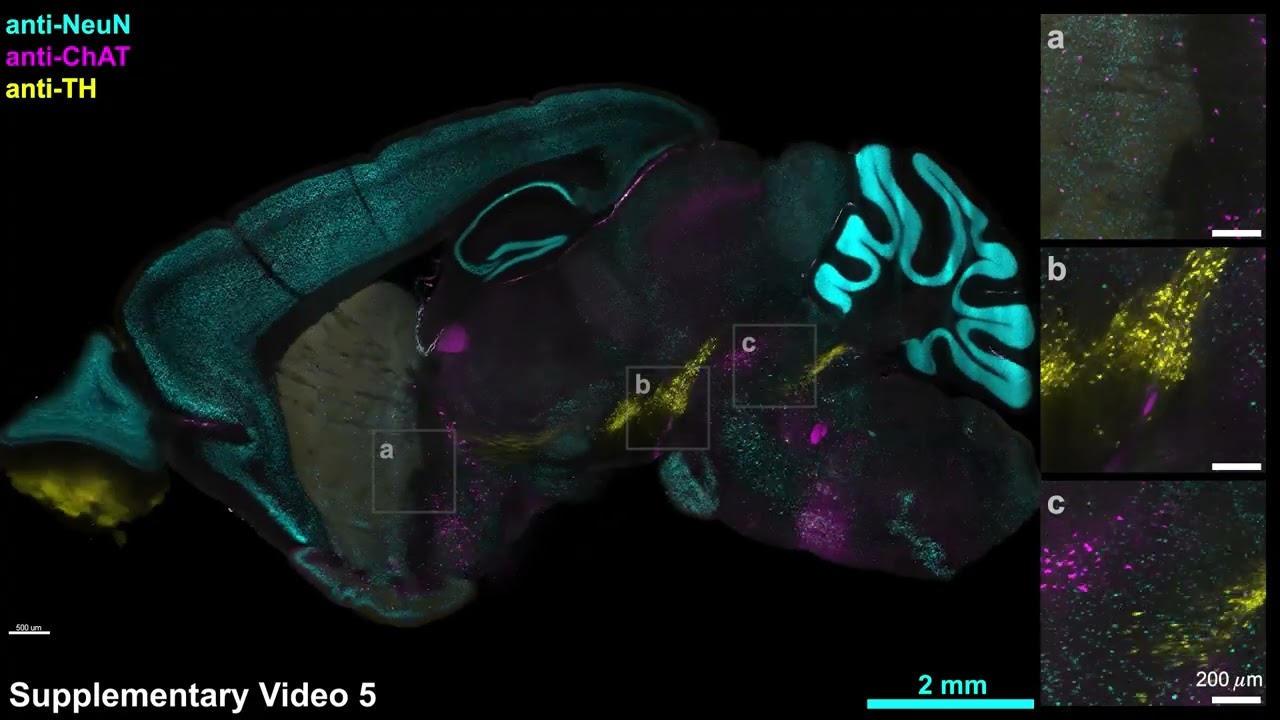
In the study the team employed both kinds of labeling simultaneously in samples. Visualizing the labels that way, they saw many examples in which antibody labeling and genetic labeling differed widely. In some areas of mouse brains, they found that two-thirds of the neurons expressing PV (a protein prominent in certain inhibitory neurons) according to antibody labeling, did not show any genetically-based fluorescence. In another example, only a tiny fraction of cells that reported expression via the genetic method of a protein called ChAT also reported it via antibody labeling. In other words, there were cases where genetic labeling both severely underreported or overreported protein expression compared to antibody labeling.
The researchers don’t mean to impugn the clear value of using the genetic reporting methods, but instead suggest that also using organ-wide antibody labeling, as eFLASH allows, can help put that data in a richer, more complete context. “Our discovery of large regionalized loss of PV-immunoreactive neurons in healthy adult mice and with high individual variability emphasizes the importance of holistic and unbiased phenotyping,” the authors write.
Or as Yun puts it, the two different kinds of labeling are “two different tools for the job.”
In addition to Yun, Park, and Chung, the paper’s other authors are Jae Hun Cho, Lee Kamentsky, Nicholas Evans, Nicholas DiNapoli, Katherine Xie, Seo Woo Choi, Alexandre Albanese, Yuxuan Tian, Chang Ho Sohn, Qiangge Zhang, Minyoung Kim, Justin Swaney, Webster Guan, Juhyuk Park, Gabi Drummond, Heejin Choi, Luzdary Ruelas, and Guoping Feng.
Funding for the study came from the Burroughs Wellcome Fund, the Searle Scholars Program, a Packard Award in Science and Engineering, a NARSAD Young Investigator Award, the McKnight Foundation, the Freedom Together Foundation, The Picower Institute for Learning and Memory, the NCSOFT Cultural Foundation, and the National Institutes of Health.
Upgrade your audio game with the Logitech for Creators Blue Yeti USB Microphone. With over 33,730 ratings and an impressive 4.6 out of 5 stars, it’s no wonder this is an Amazon’s Choice product. Recently, 5K+ units were purchased in the past month.
Available in five stunning colors: Teal, Silver, Pink Dawn, Midnight Blue, and Blackout, this microphone is perfect for creators looking to produce exceptional audio. Priced at only $84.99, it’s a deal you can’t afford to miss.
Elevate your recordings with clear broadcast-quality sound and explore your creativity with enhanced effects, advanced modulation, and HD audio samples. Order now for just $84.99 on Amazon!
Help Power Techcratic’s Future – Scan To Support
If Techcratic’s content and insights have helped you, consider giving back by supporting the platform with crypto. Every contribution makes a difference, whether it’s for high-quality content, server maintenance, or future updates. Techcratic is constantly evolving, and your support helps drive that progress.
As a solo operator who wears all the hats, creating content, managing the tech, and running the site, your support allows me to stay focused on delivering valuable resources. Your support keeps everything running smoothly and enables me to continue creating the content you love. I’m deeply grateful for your support, it truly means the world to me! Thank you!
|
BITCOIN
bc1qlszw7elx2qahjwvaryh0tkgg8y68enw30gpvge Scan the QR code with your crypto wallet app |
|
DOGECOIN
D64GwvvYQxFXYyan3oQCrmWfidf6T3JpBA Scan the QR code with your crypto wallet app |
|
ETHEREUM
0xe9BC980DF3d985730dA827996B43E4A62CCBAA7a Scan the QR code with your crypto wallet app |
Please read the Privacy and Security Disclaimer on how Techcratic handles your support.
Disclaimer: As an Amazon Associate, Techcratic may earn from qualifying purchases.

















































![[Download] Intuitive 3D Modeling | Abstract Sculpture | FLIGHT | DANA KRYSTLE](https://techcratic.com/wp-content/uploads/2025/08/1755630966_maxresdefault-360x180.jpg)







































![[DEBUT COVER] Intergalactic Bound – Yunosuke / CircusP [MIKU EXPO 10th]](https://techcratic.com/wp-content/uploads/2025/08/1755598927_maxresdefault-360x180.jpg)